Bioester in Bioscience Discipline-Past, Present and Future Trends
CurrentTrends in Biomedical Engineering & Biosciences Juniper Publishers
Authored by Mohammad Shahidul Islam*
Abstract
Bioester, as a member of life science discipline has enormous potential to be incorporated in pharmaceutical and personal care product (PPCP) system because of its increased customer demand. Bioester is produced by transesterification between two immiscible edible oil and organic alcohol phases in presence of an alkaline catalyst. The bioester thus produced has diversified area of applications and PPCP industry is one of them. This bioester has reduced viscosity (10% of neat edible oil), improved physio-chemical properties and excellent compatibility for precious applications. Due to this reason past, present and future of bioester, as a member of life science discipline is very impressive and it will develop in course of time.
Keywords: Alkaline catalyst, bioester, edible oil, life science, organic alcohol, pharmaceutical and personal care product, shea butter ethyl ester, transesterification.
Biomaterial Carriers
Bioscience or life science is derived from life or biomass substances. Biomass substance is renewable in the sense that it absorbs same quantity of carbon di oxide from atmosphere that it emits while combusted. Carbon re-fixation life span varies from few months to few years. Bioester is a transesterifying product of life science species, triglyceride. Triglyceride is produced from various energy seeds, i.e., rape, soybean, mustard, sunflower, coconut, peanut etc. seeds.
Back in 1900 century biofuel, neat peanut oil was used in fueling a car in Paris expo by Rudolf Diesel, inventor of diesel engine. At that time, Dr. Diesel quoted, biofuel may seems to be inferior for the time being because of lack of technological development but it would be valuable in powering engine system in course of time. But later on neat edible oil was found un-suitable in fuel engine system because of its high viscosity (around 45mm2/s at 40 ˚C). High viscous edible oil damages piston of fuel engine because of stocking effect.
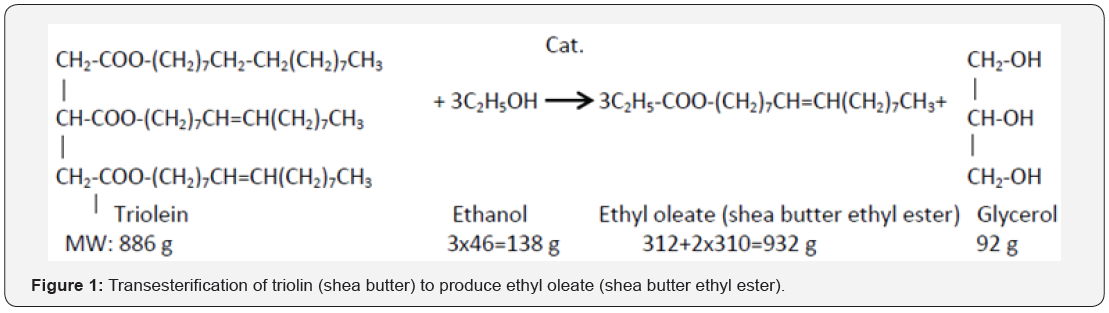
Glycerin back bone is responsible for high viscosity in edible oil. To reduce its viscosity to engine friendly level, Bioester product is developed. The underlying chemical reaction is called transesterification. In transesterification reaction, an organic alcohol (ethanol/methanol) is used to split glycerol back bone of the triglyceride. An alkaline catalyst is usually used to promote the transesterification reaction in favorable direction. Usually CSTR or plug flow turbulent reactor system is used to induce vigorous mixing between two immiscible oil and alcohol phases. Three step reactions occur to complete catalyzed transesterification reaction (Figure 1).
Before the transesterification reaction, edible oil is preheated to the boiling temperature of the organic alcohol. At the boiling temperature, triglyceride of the edible oil is substituted to diglyceride. During propagation of the reaction, diglyceride is substituted to monoglyceride. In the last step, monoglyceride is substituted to glycerol. In each step of the three step substitution reactions, equivalent mole of bioester/alkyl ester/biodiesel is produced. When the intermediate by products monoglyceride and diglyceride are produced, two phase oil-alcohol fluid systems are reduced to single phase fluid system where lower viscosity (temperature effect) and weak fluid (oil)-fluid (alcohol) phase boundary enhances oil-alcohol mass transfer. Temperature nearly to boiling point of the alcohol also influences transesterification reaction to favorable direction.
Bioester thus produced by transesterification reaction was originated back in 1900 century. But it was not introduced in diversified area of application at that time. Presently bioester is used as biodiesel, ingredient of cosmetic production, pharmaceutical application, solvent application, reagent of chemical reaction etc [1-3].
Generally methanol and ethanol are used as Solvent for bioester production. Methanol is cheap, non-renewable and toxic. Therefore it is not suitable for cosmetic and pharmaceutical applications. But due to availability and cheap alternative, it is extensively used in biodiesel production to fuel engine system. While ethanol is non-toxic, renewable and costly alternative. Therefore, it is used to produce precious bioester for cosmetic and pharmaceutical applications. Presently shea butter and palm oil are used to produce shea butter ethyl ester and palm oil ethyl ester for cosmetic and pharmaceutical applications.
Future of bioester as bioscience/life science product is enormous. Would wide population is increasing day by day at the rate of 1.12% as of 2017. Presently total population of the world is 7.5 billion as of 2017. Demand for pharmaceutical and personal care product (PPCP) for these large numbers of population is very high. Due to this reason, PPCP industry will grow exponentially to meet the increased customer demand. For this, bioester as a raw material in PPCP can play a dominant roll to meet the increased customer demand.
Finally, renewable and eco-friendly product bioester as a member of bioscience discipline opened a frontier of science in the past after its invention. It undergoes development in the present. It has enormous potential to be incorporated in the future also. As a result past, present and future trends of bioester product reveal impressiveness and it will grow exponentially in course of time for the greatest interest of mankind.
To
Know More About Current Trends in Biomedical Engineering & Biosciences Please
Click on:
https://juniperpublishers.com/ctbeb/index.php

Comments
Post a Comment